About Us
The Alagille Syndrome Alliance (ALGSA) is the leading 501c3 patient advocacy organization in the world serving families affected by Alagille Syndrome, a rare cholestatic liver disease and one of over 7,000 rare diseases in the world.
The ALGSA staff and board is made up of professionals in the ALGS community including patients, caregivers, and friends all deeply understanding of the complex and difficult nature of ALGS.
MISSION STATEMENT Mobilizing resources, facilitating connections, promoting unity, and advocating for a cure to inspire, emopower, and enrich the lives of people affected by Alagille Syndrome.”
VISION ALGS Warriors thrive in a close-knit community full of loving support, easily accessible resources, and life affirming hope.
GOALS
- Support the ALGS community by continuing our innovative efforts that emphasize education, opportunities to connect, access to resources, and individual support.
- Build capacity to increase engagement in advocacy and research, helping support development of therapies that benefit ALGS Warriors around the globe.
- Engage medical professionals to broaden knowledge of ALGS, develop guidelines for care, communicate with practitioners, and inform medical students through ALGS education.
- Offer a symposium every three years for families to access resources that meet their needs and help them thrive.
- Levereage staff resources and external relationships to stay financially healthy and sustainable.
- Implement an operational model that allows for growth and continues to encourage flexibility and responsiveness to the needs of our community.
Founder
Cindy Luxhoj
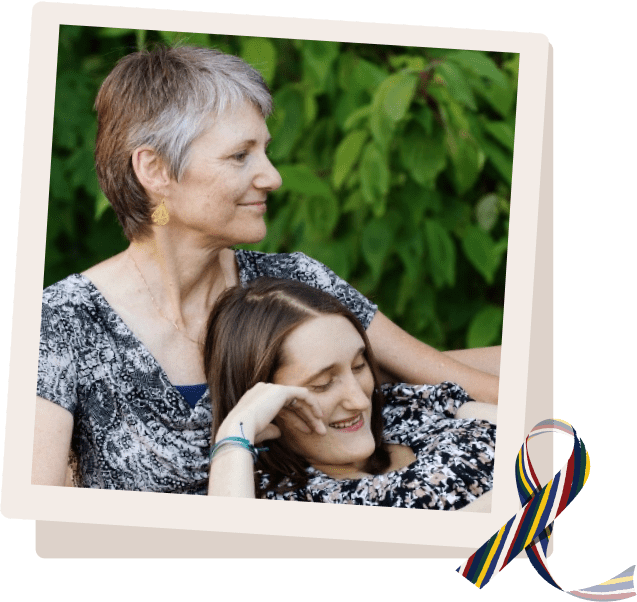


The ALGSAwarness Ribbon


YELLOW – for the liver and digestive system
RED – for the heart and circulatory system
GREEN – for the kidneys and renal system
BLUE – for the lungs and pulmonary system
PURPLE – for the brain and vascular system
CREAM/PEARL – for the skeletal system
The Alagille Syndrome Alliance awareness ribbon for ALGS was created in 2018 and reflects the significantly complex and uniquely varying severity of the disease throughout our ALGS community. This ribbon recognizes those affected by just a few organ systems and those affected by all organ systems. It is our hope that we can unite, relate and empower everyone dealing with ALGS, either mild or severe, and bring together those with significant organ representation who may not focus on ALGS as a whole but rather the organ system most affected.
We want our ALGS heart families, liver families, kidney families, and vascular families struggling through their diagnoses to know we are here fighting for them. We want to impress on our community that Alagille Syndrome cannot be treated as one single condition. Together, we can fight to find the cure through researching each organ system and supporting families who relate more to one significantly affected symptom. Our Alagille Syndrome awareness ribbon is meant to encourage our ALGS families, scientists, researchers, and donors and show the world that we cannot be separated by symptoms but rather united by uniqueness.
This multi-colored ribbon represents all of the major body systems affected by Alagille Syndrome. Each color may stand alone backed by the ALGSA. When combined, the ribbons form a strong and beautiful representation of all of our Alagille Syndrome Warriors!
As Alagille Syndrome affects so many systems, we want to spread the word to other communities. Our research could help others with quicker diagnosis and access to better treatments. If you are a part of any organizations or groups serving the communities of any of these systems, please share our information. Together, we can find better treatments and a cure for ALGS and other associated diseases!


Financial
The Alagille Syndrome Alliance is committed to the highest levels of transparency. Annually, we arrange for an external audit of our financial statements and make our financials available upon request. To verify our tax-exempt status, please use our tax ID# 93-1243619 to search.
Our Partners














Media Partners


















Staff


Roberta Smith


Cher Bork


Stephanie Mullett
Program Administrator


Karsten Baumgaertel
Scientific Research Coordinator
Board


Julia Bird
Chairman / Secretary


Todd Allen
Treasurer

Shambhavi Ravishankar


Mike LaRosa


Sean Kelley
Honorary Emeritus Board Member


Alaina Hahn
Medical Advisory Board


Dr. David Piccoli
Children’s Hospital of Philadelphia
Philadelphia, PA


Dr. Binita Kamath
Toronto, Canada


Dr. Nancy Spinner
Philadelphia, PA


Dr. Jeffrey Feinstein
Palo Alto, CA


Dr. Henry Lin
Portland, OR


Dr. Ruben Quiros
Omaha, NE


Dr. Ronald Sokol
Colorado Springs, CO


Dr. Duc Dong
Medical Discovery Institue
La Jolla, CA


Dr. Dennis Black
Memphis, TN


Dr. Philip Rosenthal
San Francisco, CA


Dr. Kathleen Loomes
Philadelphia, PA
